You have a Eriocheir sinensis H. Milne Edwards, 1853 – Chinese Mitten Crab
Synonyms: There is some confusion about the actual number of species of Eriocheir and whether some of the existing names are synonyms of Eriocheir sinensis (see below).
Common names :Chinese mitten crab, Chinese river crab, Chinese freshwater edible crab, Shanghai crab (UK, USA, CAN); Kinesisk uldhåndskrabbe (DK); Kinesisk ullhåndskrabbe (NO); Kinesisk ullhandskrabba (SE); Chinesische Wollhandkrabbe (GE); Chinese Wolhandkrab (NL); Crabe chinois, Crabe poilu de Shangai (FR, CAN); Cangrejo chino (SP); Krab welnistoreki (PL); Villasaksirapu (FI).
Systematic note: The year of description is often given as 1854. However, Clark (2006) showed that it should be 1853, and also that the family should be Varunidae rather than Grapsidae.
The most characteristic feature of adult mitten crabs is the hairy "mittens" covering the chelae of adult crabs, especially the males. The carapace grows to about 8 cm in width. It has a quadrangular outline with a couple of spines on the latero-frontal margin and some smaller spines and a median notch between the eyes. Adult males are usually larger and heavier than females and have larger claws. The colour is usually brown or greenish brown, though paler in juvenile animals. Juvenile crabs (<2 cm carapace width) do not have the hairy mittens and may be confused with other species, especially another introduced species, Rhithropanopeus harrisii (Gould, 1841), which also has white-tipped claws, but a smooth carapace margin between the eyes (see fact sheet for this species). In the native common shore crab, or green crab, Carcinus maenas (Linnaeus, 1758) the carapace is widest towards the anterior end and gradually tapers towards a narrow, rounded posterior end. In the Nordic countries there are no freshwater crabs that can be confused with the Chinese mitten crab.

Picture of Eriocheir sinensis ♀, ventral view where the broad abdomen is visible. Photo by Kathe Rose Jensen
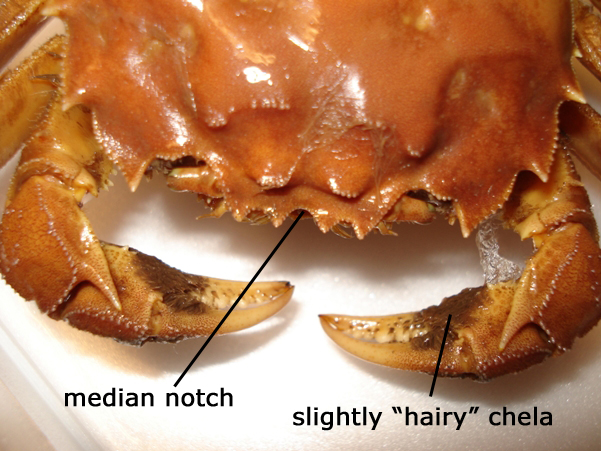
Picture of Eriocheir sinensis ♀, anterior, dorsal view where the slightly "hairy" chela are visible. Photo by Kathe Rose Jensen

Picture of Eriocheir sinensis ♂, ventral view where the "hairy" chelipeds and the narrow abdomen are visible.
Photo by Kathe Rose Jensen

Picture of Eriocheir sinensis ♂, dorsal view where the "hairy" chelipeds are visible. Photo by Kathe Rose Jensen
Native area: Eriocheir sinensis is a native of Eastern Asia. In coastal waters its range extends from Hong Kong in the south to North Korea (Guo et al., 1997; Herborg et al., 2003) or possibly, depending on synonymies, from Taiwan to Vladivostok in Russia (Gollasch, 2006a). It may migrate upstream more than 1000 km through rivers and canals. In eastern Asia several species of Eriocheir exist (Guo et al., 1997; Zhao et al., 2002; Chu et al., 2003; Tang et al., 2003), though some of these have been listed as synonyms of E. sinensis (Gollasch, 2006b; Kelly & Maguire, 2009). Aquaculture in the native region may have caused introductions of populations genetically different from native populations, though hybridization between species is apparently not possible (Guo et al., 1997).
History of invasion: The first record in Europe is from 1912 when one specimen was found in the River Aller in Germany (Jensen, 1936; Dittel & Epifanio, 2009). Next it was found in the River Elbe in 1914, and here a population burst was observed in the 1930s (Herborg et al., 2003). Presently there is a reproducing population in this river (Montú et al., 1996). The first record from the Baltic coast of Germany is from 1926 (Ojaveer et al., 2007). The first Danish record is from 1927 (Jensen, 1936) and later records have been summarized by Rasmussen (1987, 1993) and Tendal (2001, 2003, 2008). Ovigerous females have only been found on a few occasions in Danish waters, and in all of these the eggs were undeveloped or decomposing (Jensen & Knudsen, 2005). Hence it cannot be considered established in Danish waters, though recently egg-carrying females have been observed in Ribe Å, a stream entering the Wadden Sea, where salinity may be high enough for reproduction (Tendal, 2008). In Norway it only occurs in the southern part, where it was first found in 1976 (Hopkins, 2001). In Sweden the first record appears to be from Bråviken, a fjord south of Stockholm, in 1932 (Lundin et al., 2007). In 1933 it was found in freshwater in Lake Mälaren (Josefsson & Andersson, 2001). Presently it is found from the Bay of Bothnia in the Baltic to Gothenburg along the west coast as well as in the large lakes (Främmande arter, 2006; Lundin et al., 2007). Populations have been increasing since 2000 (Lundin et al., 2007). It is noteworthy that molecular studies have shown that the population in Lake Mälaren is identical to populations from rivers of the Netherlands and Germany (Herborg et al., 2007), supporting the hypothesis that Baltic mitten crabs have migrated from the North Sea as larvae. In Poland it was first recorded in 1928 from the Szczecin Lagoon, where it spread upstream the river Oder (Czerniejewski and Wawrzyniak, 2006). Egg-carrying females have been found in Polish coastal waters (Normant et al., 2002), but reproduction probably cannot be completed in the Baltic (Ojaveer et al., 2006). In 1932 it was recorded from the Czech Republic to where it had migrated up the river Elbe, a total of 700 km (Herborg et al., 2003). It has been recorded from Lithuania since 1935 (Bacevičius & Gasiūnaitė, 2008), Latvia since 1932, Estonia and eastern Gulf of Finland since 1933 (Herborg et al., 2003; Ojaveer et al., 2007). Although it has been known from coastal Finland since 1933, it was only found in Finnish inland waters in 1999 (Valovirta & Eronen, 2000). The same has been observed at the Russian Baltic Sea coast and inland waters (Panov, 2006).
In the Netherlands the first specimens were caught in 1929 in the Ems-Dollard estuary and by 1935 it had spread to all of the country (Wolff, 2005). It was first recorded from France in 1930 and from Belgium in 1933 (Herborg et al., 2003). In France it slowly progressed along the coast of the English Channel, and it reached the river systems of the Bay of Biscay during the 1950s (Herborg et al., 2005). Through rivers and canals it reached the French Mediterranean coast in 1959 (Herborg et al., 2003). The first record from the UK is from 1935 in the River Thames at a power station (Clark et al., 1998; Herborg et al., 2005). Next it was found in 1939 in the Humber River, but it was not permanently established until 1973. Until 1996 it only occurred in the Humber and the Thames (Clark et al., 1998), but since 1997 it has spread further north along the North Sea coast and also to the south coast. Furthermore the rate of dispersal has increased since the late 1990s (Herborg et al., 2003, 2005). In Ireland it has only been found since 2006 (Minchin, 2007). Most recently it has been found in Portugal (Cabral & Costa, 1999). In most European countries there has been an early phase of establishment with slow secondary dispersal, followed by periods of rapid dispersal, several hundred km per year and massive population outbreaks. In some cases, however, human assistance is suspected for some of the most remarkable "leaps" in dispersal (Herborg et al., 2005). Molecular studies show that European populations originate from several invasions (Hänfling et al., 2002).
Eriocheir sinensis has also been introduced to the Black Sea and the Sea of Azov, and more recently to the Caspian Sea (Robbins et al., 2006). It has been found in the river Danube (Paunovic et al., 2004) as well as the Volga (Shakirova et al., 2007). Finally, it has been collected from the Arabian Gulf, Iraq (Clark et al., 2006).
On the east coast of North America E. sinensis was first reported in 1965 from the North American Great Lakes (Ruiz et al., 2006; de Lafontaine et al., 2008), but breeding apparently does not occur (Dittel & Epifanio, 2009). Further south it was reported once from the Mississippi River delta in 1987 and in the Chesapeake Bay in 2005 (Ruiz et al., 2006), but it is unknown if the species is reproducing in these areas. In 2004 the first mitten crab was found in St. Lawrence River and in 2006 in the St. Lawrence Estuary. Molecular studies show the presence of genotypes also found in Europe, western USA and China. Hence the source population cannot be identified (de Lafontaine et al., 2008), though based on shipping activities, European origin seems most likely (Tepolt et al., 2007).
The Chinese mitten crab has also invaded San Francisco Bay on the west coast of the USA. Here it was first recorded in 1992, and a breeding population is established (Cohen & Carlton, 1997; Rudnick et al., 2003). Molecular studies show that only one genotype occurs here, and it is identical to a genotype found in Europe but not in China (Hänfling et al., 2002).
Vector: Possibly the Chinese mitten crab was brought to Europe as larvae in ballast water (Herborg et al., 2003). Molecular studies show that several introductions to Europe have occurred (Hänfling et al., 2002). Within Europe ballast water may also have been involved, but also accidental transport with live mussels for aquaculture is suspected (Herborg et al., 2005).
The Chinese mitten crab is omnivorous and rather non-selective, feeding on aquatic plants and invertebrates (Paunovic et al., 2004). Prey organisms may include commercially important or even endangered species (Dittel & Epifanio, 2009). It is generally a freshwater species, which only migrates to the sea for reproduction (Normant et al., 2000). It is capable of migrating about 1500 km upstream from the North Sea to the Baltic Sea (Ojaveer et al., 2007). Eriocheir sinensis is an osmoregulator in freshwater, but osmoconformer in seawater (Dittel & Epifanio, 2009). Juveniles may occur in extremely high numbers, and their burrowing activities cause severe damage to river banks (Herborg et al., 2003; Rudnick et al., 2005a). Burrows may be highly complex, composed of interconnecting tunnels and chambers (Rudnick et al., 2005a). The hairy "mittens" can form habitats for a number of small organisms, but so far no alien species have been found (Normant et al., 2007).
Reproduction: This species has a complex life cycle involving migrations between freshwater and the sea. Mitten crabs need a salinity of at least 15 ppt for reproduction (Anger, 1991). Mating takes place in estuaries, i.e. in brackish water, and animals have hard shells when they mate (Herborg et al., 2006). Fertilized eggs are released within 24 hours of mating. Females carry up to one million, approximately 350 µm diameter eggs (Rudnick et al., 2003; Dittel & Epifanio, 2009), and one female may release three batches of eggs in one mating season (Herborg et al., 2006). Brooding time is up to two months (Dittel & Epifanio, 2009). Larvae hatch as Prezoea and go through five Zoea stages, which can be extended with a sixth Zoea if conditions are unfavorable. Zoea V metamorphoses into a Megalopa stage which finally metamorphoses into juvenile crabs (Montú et al., 1996). Larval development requires temperatures above 12° C and tolerance for low salinities increase with higher temperatures (Anger, 1991). Larval stages are described and figured in Hinrichs & Grell (1937), Panning (1939), Montú et al. (1996) and Dittel & Epifanio (2009). Zoea I is tolerant of different salinities, whereas the other Zoea stages are rather stenohaline, requiring salinities of 25 ppt (Anger, 1991) and the Megalopa is again euryhaline (Anger, 1991). This reflects the fact that larvae hatch in brackish water, migrates to the sea for further development and return to eastuaries as Megalopas. Laboratory experiments indicate that the larvae take about 3 months from hatching till the Megalopa stage is reached (Anger, 1991). Sexual maturation apparently takes longer in European populations (3-5 years) than in the native area (1-3 years) (Herborg et al., 2005). Mating season differs little between native and introduced ranges, occurring between November and March in Chinese mitten crabs, from October to January in German, and from September to February in British specimens (Herborg et al., 2006). Crabs from California begin mating in November.
Drawings of zoea and megalopa larvae
Ecological: The crab burrows extensively along river banks (Herborg et al., 2003; Rudnick et al., 2005a; Gilbey et al., 2008). Juvenile crabs may displace juvenile Carcinus maenas (Gilbey et al., 2008). In European freshwaters there are no crustacean competitors, but in California mitten crabs in freshwaters compete for space with native crayfish (Dittel & Epifanio, 2009). In its native area it is intermediate host of the parasitic lung-fluke Paragonimus westermani (Kerbert, 1878) which has mammals, including humans, as final host. This parasite is a big problem in several Asian countries, including Korea, China and Japan, but so far has not been detected in the introduced range.
Economical: E. sinensis is considered one the 100 worst invasive species by the IUCN Invasive Species Specialist Group (ISSG). It interferes with recreational fishing, feeding on the bait and fish from fish traps, damaging fish captured in gill nets, and also damaging the fishing gear (Rudnick et al., 2005b). Its burrowing activities cause erosion or destabilization of river banks (Gilbey et al., 2008). In its native area it is considered a delicacy, supporting an annual billion-dollar fishery (Herborg et al., 2005; Sui et al., 2009), and eating it has also been suggested as a way of exterminating it in its invasive regions (see: http://www.nhm.ac.uk/about-us/news/2009/february/thames-alien-crab-safe-to-eat27526.html). In its native area wild populations have been overexploited and stock enhancement and aquaculture from wild-caught megalopa larvae is used (Sui et al., 2009). Culture method is described by FAO (http://www.fao.org/fishery/culturedspecies/Eriocheir_sinensis/en).
The importance of the introduction of Eriocheir sinensis was realized early on. In Germany two entire journal volumes were dedicated to this species (Peters et al., 1933; Klapp, 1938). During the 1930s the German government supported attempts to eradicate the mitten crab from several rivers where fishery had been disturbed. Traps were constructed to attract and catch crabs at bridges, sluice-gates and other man-made constructions, but although more than 100,000 kg were caught every year from 1933 through 1937, crabs are still present and thriving.
More recent management or action plans are available for the USA (http://www.anstaskforce.gov/Chinese-mitten-crab-plan2-02.pdf), Ireland (Kelly & Maguire, 2009), and New Zealand, where the species has not (yet?) been found (http://www.biodiversity.govt.nz/pdfs/seas/chinese_mitten_crab_action_plan.pdf). Risk assessments have been carried out for Oregon, USA and for Canada (Therriault et al., 2008).